What is a Natural Resource?
By: Matt Brinckman (email)
What is a Natural Resource?
- a naturally occurring substance that is considered valuable in it’s relatively unmodified or natural form.
- Examples of a natural resource: water, wood, oil, and wildlife.

What is the difference between a renewable and a nonrenewable resource?
- Forests can provide timber, recreation, wildlife habitat, water cycling, and non-wood forest products like coffee, fruit, nuts, and even rubber.
So what is it that allows forests to renew themselves???
- PHOTOSYNTHESIS!!! (the synthesis of sugar from light, carbon dioxide, and water with oxygen as a waste product.)
- Photosynthesis broken down: Photo = Light Synthesis = putting together
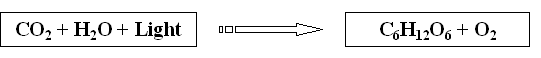
The Photosynthesis Equation:
CO2 = Carbon dioxide C6H12O6 = Carbohydrate (sugar)
H2O = Water O2 = OxygenSo why is Photosynthesis Important?
- All life on earth is dependent on the sun for energy. Photosynthesis is the only natural process that directly captures energy from the sun! All plants, algae, and some bacteria and protists go through photosynthesis.
Carbon Fixation
- This is simply the process of extracting carbon from carbon dioxide molecules in the air and fixing them into a carbohydrate compound through photosynthesis.
- To imagine carbon fixation at work, follow a single CO2 molecule from your own breath as it floats towards a tree, taken in by the leaves, put through photosynthesis and processed into wood. Your carbon molecule from your breath will now be unavailable to the atmosphere until it is released through combustion.
Introduction to the combustion/respiration equation
- Whenever organic materials are combusted or decomposed, the chemical reaction that takes place is simply the reverse of the photosynthesis equation.
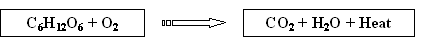
- Now if you can still imagine the carbon molecule that you breathed out earlier which was then fixed into a tree, imagine that tree burning up in an intense forest fire. The carbon molecule that you once breathed out is now released from the wood and made available for further uptake in the atmosphere.
- To see the process of photosynthesis illustrated in an experiment click on this link to learn more from David A. Klingbiel.
Carbon Cycling and Global Warming
- Global levels of carbon dioxide have been steadily raising for the last 50 years. Higher levels of carbon dioxide help contribute to the “greenhouse gas effect”. This is defined as the process in which the absorption of infrared radiation by an atmosphere warms a planet. To see the Mauno Loa graph of the rising carbon dioxide levels click on this link. This graph also shows the annual rise and fall of Carbon dioxide because of reduced photosynthetic activity during the winter months of the northern hemisphere.
- Greenhouse gasses (gaseous components of the atmosphere that contribute to the greenhouse effect) are not all bad. There has always been a balance of these gasses that keep our atmosphere stable. In fact, if there were no greenhouse gasses, the earth would be colder by over 80 degrees Fahrenheit!!! It is maintaining this balance that some are worried about. It will be interesting to see how trees, plants, carbon fixation and photosynthesis will play a role in dealing with carbon dioxide levels in the future.